S. Q. Saikat3, A. M. Selim4, J. Kessi1, E. Wehrli2, and K. W. Hanselmann1
| 1 | Microbial Ecology Group, Institute for Plant Biology, University of Zürich. Zürich, Switzerland |
| 2 | Laboratory for Electron Microscopy, ETH-Zentrum, CH-8092 Zürich, Switzerland |
| 3 | School of Environmental and Applied Sciences University of Derby, Derby, UK |
| 4 | Dept. of Forest Mycology and Pathology, SLU, Uppsala, Sweden |
E-mail address: Jkessi@botinst.unizh.ch
We present evidence that bacterial communities enriched from aquifer sediments from Bangladesh mediate arsenic transformations. Bacteria from six different aquifers were cultured in the laboratory. Anoxic cultures from four out of six sediments tested exhibited high arsenate-reducing activity, while cultures from only one out of three sediments were shown to have high arsenic sulfide-oxidizing activity when incubated under oxic conditions. Our results suggest that sediment bacteria may transform arsenic under natural conditions and thereby play a pivotal role in the mobilization and immobilization of arsenic compounds in some arsenic-contaminated aquifers.
The arsenate-reducing enrichment cultures were composed of mostly rod-shaped or curved cells. Fluorescence In Situ Hybridization (FISH) indicated that most bacteria from one of the sediments belong to the eubacterial group, some of which are also members of the d-group of the Proteobacteria.
Electron micrographs of two of the anoxic cultures that exhibited high arsenate-reducing activity showed cells with both high and low electron densities. In one culture, particles appeared to be attached to the surfaces of most of the cells, while in another culture, a number of such particles were apparently floating in the culture medium. Transmission electron microscopy-energy dispersive X-ray analysis (TEM-EDXŲanalysis) of both particles and cells is in progress in order to determine whether or not they may contain arsenic.
Arsenic in drinking water is a serious public health problem in developing and industrialized countries alike where geogenic arsenic is present at elevated concentrations in soils and sediments. The problem is particularly acute in Bangladesh where 77 million people consume arsenic-contaminated ground water (Wilson, 2000).
Two main hypotheses have been formulated to explain the release of arsenic oxyanions into ground water: (a) A change in the redox conditions in the ground water due to over-exploitation of wells, leading to the oxidation of insoluble arsenic sulfides, resulting in the formation of the more soluble arsenic oxyanions (Chakraborti, 2000). (b) The release of arsenate into ground water through reduction of insoluble iron oxyhydroxides which contain arsenate in their amorphous matrix (Nickson et al., 1998)
Over the past decade dissimilatory arsenate reduction involving energy conservation has been described for many arsenate respiring organisms such as: Geospirillum arsinophilus (Ahmann et al., 1994), Geospirillum barnesii (Laverman et al., 1995), Chrysiogenes arsenatis (Macy et al., 1996), Desulfovibrio spp and Desulfomicrobium spp (Macy et al., 2000). This reaction can be described for neutral pH by the following equation:
| HAsO4-2 + H2AsO4- + 7H+ + 4e- |
|
2H3AsO3 + 2H2O | (1) |
| Arsenate (As(V)) | Arsenite (As(III)) |
In Desulfotomaculum auripigmentum (Newman et al., 1997) the end product of arsenate reduction was found to be arsenic trisulfide (As2S3). Transformation of arsenite to As2S3 was shown to be performed by this organism in the presence of the hydrogen sulfide originated from sulfate reduction.
Certain aerobic bacteria oxidize arsenite to arsenate (Santini et al., 2000). This reaction can best be described by equation (2)
| 2H3AsO3 + O2 |
|
HAsO4-2 + H2AsO4- + 3H+ | (2) |
| Arsenite (As(III)) | Arsenate(As(V)) |
However, no study has yet been published on the potential for changes in arsenic speciation by sediment microorganisms in ground water.
We have enriched for arsenic-transforming bacteria from six different arsenic-contaminated aquifer sediments of Bangladesh. Our investigations have focused on:
Anoxic liquid culture media were inoculated with groundwater sediments obtained from six different aquifers in Bangladesh that are located in various regions of the country (Fig. 1). The culture medium contained 10 mM lactate as a carbon source and a mixture of salts, trace elements and vitamins as described for growing sulfate-reducing bacteria (Newman et al., 1997). Cysteine HCl served as a reductant and resazurin as a redox indicator. The pH of the cultures was matched with the pH of the sediments, which ranged from pH 4-9 (Table 1). The cultures vessels were inoculated with about 10% (w/v) of sediment material; the same percentage (v/v) was used for making subcultures. Several subcultures were established in the presence of 5 mM arsenate in an effort to enrich for arsenate-tolerant bacteria. All enrichment cultures and subcultures were maintained at room temperature.
Arsenate-reducing activity of sediments from locations 3, 4 and 6 was exhibited in the first subcultures (B subcultures; Fig. 2). Sediment 3 subcultures exhibited very gradual arsenate reduction, with less than 10% of the arsenate reduced in seven days. Subcultures from sediment 4 reduced about 30% of the initial arsenate in the same period of time. Subcultures from sediment 6 transformed all of the added arsenate within seven days.
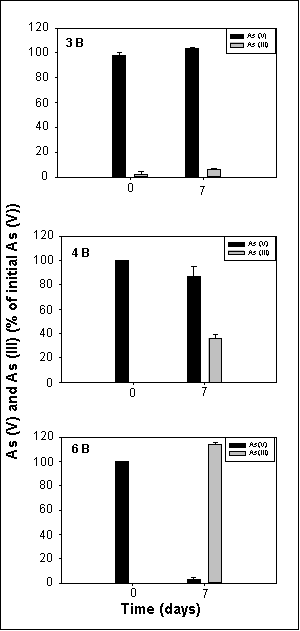 |
Figure 2. Reduction of arsenate [As(V)] to arsenite [As(III)] in the first subcultures (designated as B subcultures) made from sediments 3, 4 and 6. The cultures were grown in a liquid medium under anoxic conditions at room temperature. The cultures were amended with 5 mM arsenate upon inoculation, and arsenate and arsenite were first determined seven days after inoculation according to the method of Johnson and Pilson (1972). Culture samples were centrifuged at 15'000 g for 10 min. before arsenic determination. Data represent the mean of analyses of 2 different cultures. |
The arsenate-reducing activity of sediments 1, 2, and 5 (Fig. 3) were determined in a third set of subcultures (D subcultures). All three types of subcultures exhibited very high arsenate-transforming activity, leading to 80-100% transformation of arsenate to arsenite within seven days.
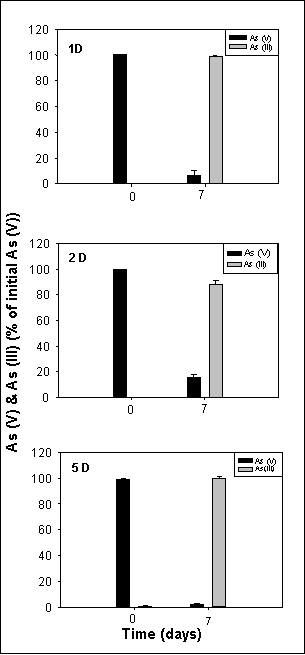 |
Figure 3. Reduction of arsenate to arsenite in the third subcultures (D subcultures) obtained from sediments 1, 2, and 5. Culture conditions, as well as arsenate and arsenite determinations, were as described for Figure 2. Data represent the mean of analyses of 2-4 cultures. |
A few days after reduction of arsenate to arsenite was complete, the cultures originating from sediments 1 and 5 produced a yellow precipitate. SEM-EDX analysis of the solid phase of such a culture harvested one week after completion of arsenate reduction, showed large signals for arsenic and sulfur, suggesting it to contain arsenic-sulfide. It also shows a large signal for silicon, indicating a contamination by sediment particles (Fig. 4A). In the cultures obtained from sediment 2, the yellow precipitate appeared about three weeks after arsenate reduction was complete. SEM-EDX analysis of the solid phase taken a few days after arsenate reduction was complete showed traces of arsenic but no sulfur. The most important peaks characterizing the solid phase of this culture were for Mg, Al, Si, K, and Fe (Fig. 4B), which may simply reflect the initial composition of the aquifer sediment.
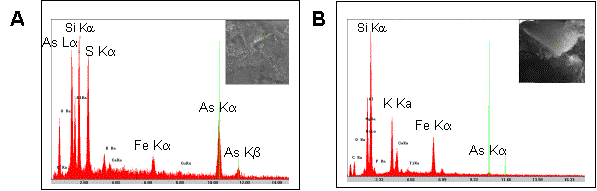 |
| Figure 4. SEM-EDX analyses of the solid phase from the second set of subcultures (C subcultures) grown under anoxic conditions and taken a few days after arsenate reduction was complete. (A) Yellow precipitate from a subculture of sediment 1 has large signals for As (La, Ka and Kb) as well as for S (Ka), suggesting it to contain arsenic sulfide. It also shows a relatively large signal for silicon indicating that the yellow precipitate may also contain sediment particles. (B) The gray solid phase from a subculture of sediment 2 only shows a very low signal for arsenic (green cursor), but large signals for silicon, potassium and iron, indicate that most of this solid phase is probably formed by aquifer sediment particles. Note the relatively large signal for iron in both sediment samples, indicating high iron concentrations in both sediments. |
Conical flasks containing the same culture medium described for anoxic cultures were inoculated with about 10% (w/w) of sediments 1, 2 and 5 and placed on a rotary shaker at 190 rpm at room temperature for 21 days. The cultures were amended with 5 mM arsenic trisulfide; transformation activity was determined by measuring arsenite and arsenate concentrations 7, 14 and 21 days after inoculation. Controls were made with cultures grown under the same conditions but by omitting arsenic sulfide and by amending sterilized cultures with 5 mM arsenic sulfide. Sterilization was achieved by autoclaving at 123°C for 12 min.
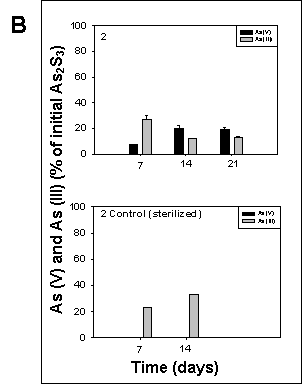
All cultures amended with arsenic sulfide exhibited arsenic sulfide-transforming activity. In sterilized controls, only arsenite could be detected, while arsenate was present in all cultures containing living sediment 7 days after inoculation. Cultures from sediment 5 exhibited the highest arsenic sulfide oxidation activity, resulting in 100% arsenic sulfide converted to arsenate after 21 days of incubation (Fig. 5).
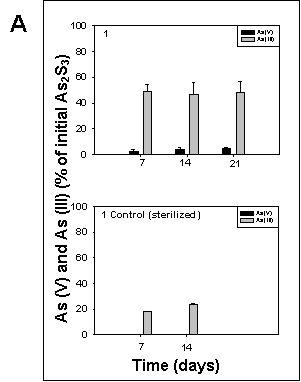 |
 |
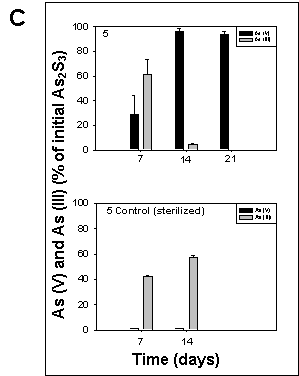 |
Figure 5. Oxidation of arsenic sulfide in cultures of sediment 1, 2, and 5 grown under oxic conditions at room temperature on a rotary shaker at 190 rpm. The culture medium was the same as described for sulfate reducing bacteria by Newman et al., (1997). The cultures were amended with 5 mM arsenic trisulfide at inoculation time. Arsenate and arsenite were determined according to the method of Johnson and Pilson (1972). Controls contained autoclaved sediment. The data represent the mean determination of 2 different cultures. |
The first indication we had about the types of bacteria involved in arsenate (As(V)) transformation was suggested by the formation of a dark precipitate in the arsenate-free cultures a few days after inoculation, which is characteristic of sulfate-reducing activity.
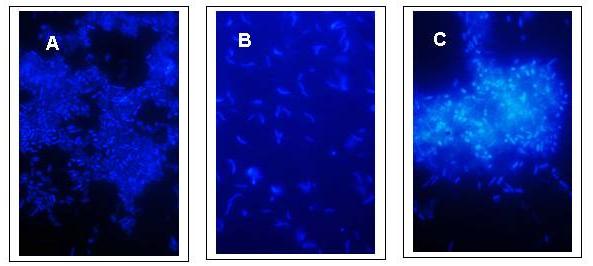
The shapes of the bacteria from the third set of subcultures from sediments 1, 2 and 5 were observed by fluorescence microscopy after DAPI staining (Fig. 6).
 |
|
Figure 6. DAPI (diamidino-2-phenyl-indole-dihydrochloride) staining of arsenate tolerant organisms from the third set of subcultures (D subcultures) grown under anoxic conditions in the culture medium described for sulfate reducers by Newman et al. (1997); culture conditions as described in the legend to Figure 2. The bacteria were fixed in 5% glutaraldehyde before addition of DAPI for a final concentration of 10 mg/ml. (A) From sediment 1. (B) From sediment 2. (C) From sediment 5. |
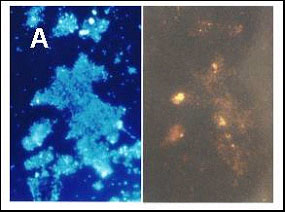
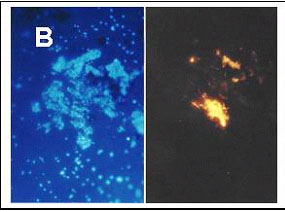
A more precise characterization of the microbial community was made using the fluorescent in situ hybridization (FISH) method in an effort to determine whether or not the bacteria from the cultures showing high arsenate-transforming activity were sulfate reducers. As indicated in the literature, most such organisms belong to the d-group of the Proteobacteria (Madigan et al., 2000); however, four different FISH probes are needed for definitively labeling all members of this group. We selected two probes specific for rod-shaped sulfate reducers. A third probe labeling the whole group of the Eubacteria was also used (Figs. 7A and 7B).
 |
 |
|
Figure 7. Staining of a fourth subculture (E subculture) from sediment 2. Comparison between DAPI staining and FISH (fluorescence in situ hybridization) on the same samples. Growth conditions as described for Figure 2. (A) Identification of Eubacteria: on left DAPI staining; on right, staining with the corresponding FISH probe. (B) Identification of bacteria from the d-group of the Proteobacteria: on the left, DAPI staining; on the right, staining with a corresponding FISH probe. |
|
While the original color of the sediments was yellow-gray to dark gray (table I), some sediment cultures without added arsenate turned black within a few days after inoculation, suggesting that iron sulfide was precipitated, due to sulfate-reducing activity. Interestingly, in the D subcultures from sediments 1 and 5, which represented the highest arsenate-reducing activity we observed (Fig. 3), no black precipitate was ever observed in the presence of added arsenate, which may indicate that arsenate, not sulfate, was the preferred electron acceptor in these cultures. In the D subcultures from sediment 2, which exhibited 80-85% arsenate reduction within 7 days (Fig. 3), the appearance of a black precipitate was independent of the presence of arsenate, which suggests that sulfate reduction is not entirely inhibited by arsenate. The cultures from sediments 3 and 4, exhibiting the lowest arsenate-reducing activity, also did not produce any black precipitate up to several weeks after inoculation. These observations suggest that sulfate reducers may be responsible for arsenate reduction in the arsenate-transforming sediments.
In the second set of subcultures (C subcultures) from sediments 1 and 5, which showed rapid arsenate-reducing activity, most bacteria were rod-shaped (Figs. 6A and 6C). The C subcultures from sediment 2, which also exhibited good arsenate-reducing potential, harbored organisms that had predominantly curved or spiral morphology (Fig. 6B).
Most organisms from a fourth set of subcultures (E subcultures) from sediment 2 yielded a signal with the FISH probe identifying them as Eubacteria (Fig. 7A). Upon the addition of another probe, a few bacteria were deemed members of the d-group of the Proteobacteria (Fig. 7B). FISH investigations of the E subcultures from sediment 1 gave only a very weak signal with each probe (photos not shown).
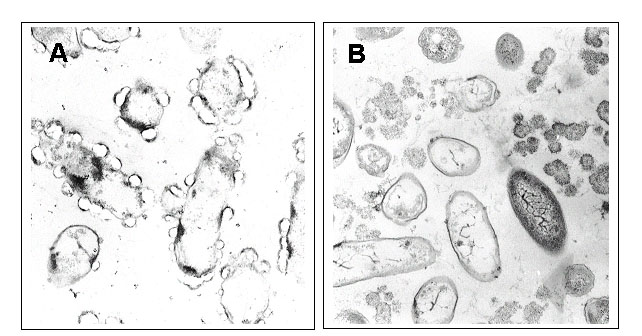
Transmission electron microscopy (TEM) has been used for determining the physiological mechanism of detoxification of arsenate in anoxic cultures. The cultures were harvested 6 days after inoculation when arsenate and arsenite concentrations indicated that arsenate reduction was complete. The cells were fixed in 2.5% aqueous glutaraldehyde and embedded in agarose. Agar blocks were fixed with OsO4, dehydrated and embedded in Epon-araldite. Cut sections were contrasted with uranyl acetate and lead citrate as described by Hess (1966). Electron micrographs with a 55‚000 fold magnification were observed (Fig. 8).
 |
|
Figure 8. Electron micrographs from representative organisms grown under anoxic conditions as described in the legend for Figure 2. The cultures were sampled 6 days after inoculation and had completed the reduction of arsenate to arsenite. (A) Fourth subculture from sediment 1. (B) Fourth subculture from sediment 5. |
The fourth set of subcultures (E subcultures) from sediments 1 and 5, showing the highest arsenate-reducing activity (Fig. 3), were observed with TEM. In the enrichment culture originating from sediment 1, most organisms were rod-shaped. Most cells appeared transparent and were surrounded by particles forming clear zones on the cell surface (Fig. 8A). Very few free particles were present in the culture medium.
In the fourth subculture from sediment 5, most organisms appeared rod-shaped to spindle-shaped (Fig. 8B). Some cells appeared dark with a few electron-dense particles inside; other organisms were transparent. Numerous, free electron-dense particles were present in the culture medium, suggesting the production of an insoluble form of arsenic.
Cultures from four of the six sediments investigated exhibited a high level of arsenate-reducing activity, while cultures from one of three sediments tested exhibited a high arsenic trisulfide oxidation activity. These results suggest that sediment bacteria may play an important role in the transformation of arsenic species and that this activity depends on the prevailing redox conditions. Under anoxic conditions, sediment bacteria may reduce arsenate to arsenite and arsenic sulfide, leading to the precipitation of the latter and the disappearance of arsenic oxyanions from the ground water. Under oxic conditions, sediment bacteria may release arsenite from arsenic sulfide complexes and oxidize it to arsenate, thereby releasing arsenic oxyanions into the aquifer.
Transformation of arsenic sulfide to arsenite in sterilized cultures could be due to a chemical transformation of arsenic sulfide in the presence of oxygen. We also observed a correlation between the arsenic trisulfide-transforming activity of active cultures and that of the sterilized cultures, which suggests that some of the bacteria mediating the transformation of arsenic trisulfide to arsenite may have survived sterilization. To our knowledge, these results constitute the first evidence of the mediation by bacteria of the transformation of arsenic species in aquifers from Bangladesh.
No correlation could be found between depth of sediment sampling and microbially-mediated arsenic transforming activity of the cultures. Sediment from location 6, withdrawn at a depth of 800 feet, displayed an arsenate reducing activity as high as that of sediment 1, 2, and 5, which were taken at a depth between 80 and 100 feet (Fig. 2, Fig. 3, Table 1). Sediments from locations 3 and 4 also taken at a depth of 80 and 100 feet, respectively, showed clearly weaker activity than sediments 1, 2, and 5 (Fig. 3 and Table 1).
The large differences in the efficiency of sediments to transform arsenic species could be due to differences of bacterial activities towards arsenic, but also to long term storage of the sediments or inadequate culture conditions, which could have adverse effects on naturally active bacterial communities.
FISH experiments using an enrichment culture of arsenate-transforming organisms derived from sediment 2 indicated that most of the bacteria of the culture belong to the Eubacteria. FISH experiments also showed that some bacteria in sediment 2 are members of the d-group of the Proteobacteria. The very low signal shown by the probes for both Eubacteria and d-Proteobacteria in the enrichment cultures from sediment 1 may have been due to the presence of a number of particles attached to the cell surfaces, which may decrease the permeability of the cell walls (Fig. 8A). FISH experiments will have to be repeated with cultures deprived of arsenic in order to test this hypothesis.
In the electron micrographs of subculture E from sediment 1 (Fig. 8A), electron-dense zones inside some of the cells may indicate the presence of arsenic in the cytoplasm. The particles surrounding most cells may contain arsenic compounds excreted by the cells and attached to the cell surfaces. The transparent appearance of these particles may be due to a loss of arsenic during preparation of the slices, thus presenting a „holeš in the place of the arsenic particles.
A similar detoxification process may be performed by cells from subculture E from sediment 5 except that arsenic excreted by the cells is no longer bound to the cell surface, but rather forming particles that appear to be freely floating in the culture medium (Fig. 8B). The differences observed between arsenate-reducing bacteria from sediment 1 and those from sediment 5 indicate that they belong to different species. The transformation of arsenate to arsenite and arsenic sulfide performed in two distinct steps, as well as the larger dimension of the particles surrounding the cells, indicate that the arsenate tolerant bacteria from the aquifer sediments of Bangladesh are different from the arsenate-reducing Desulfotomaculum auripigmentum described by Newman et al. (1997).
More accurate information about the arsenate detoxification mechanism in the arsenate-reducing organisms from sediments 1 and 5 would require TEM-EDX analysis of the cultures, thus permitting a precise localization of arsenic inside and outside of the cells.
This work was supported by the Swiss Agency for Development and Cooperation. We thank H. P. Gautschi of the Laboratory of Electron Microscopy, University Hospital, Zürich, Switzerland, for SEM-EDX-analysis and Dominique Grüter from our Institute for his kind help in FISH experiments. We also thank Prof. P.J. Colberg from the University of Wyoming, Laramie, USA, for her most valuable help in the correction of the manuscript.
Ahmann, D., A.L. Roberts, L.R. Krumholz, and F.M.M. Morel. 1994. Microbe grows by reducing arsenic. Nature 371:750
Chakraborti, P. 2000. Letter sent to website. http://phys4.Harvard.edu/~wilson/arsenic_project_countries.html
Hess, W. M. 1966. Fixation and staining of fungus hyphae and host plant root tissues for electron microscopy. Stain Technol. 41:27-35.
Johnson, D. L., and M. E. Q. Pilson. 1972. Spectrophotometric determination of arsenite, arsenate and phosphate in natural waters. Anal. Chem. Acta 58:289-299.
Laverman A.M., J. Switzer Blum, J.K. Schaefer, E.J.P. Phillips, D.R. Lovley and R.S. Oremland. 1995. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl. Environ. Microbiol. 61:3556-3561
Macy, J. M., K. Nunan, K. D. Hagen, D. R. Dixon, P. F. Harbour, M. Cahill, and L. I. Sly. 1996. Chrysiogenes arsenatis gen. sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int. J. Syst. Bacteriol. 46:1153-1157
Macy, J. M., J. M. Santini, B. V. Pauling, A. H. O‚Neill, and L. I. Sly. 2000. Two new arsenate/sulfate-reducing bacteria: mechanisms of arsenate reduction. Arch. Microbiol. 173:49-57
Madigan, M. T., J. M. Martinko, and J. Parker. 2000. Brock. Biology of Microorganisms. 9th edition, pp. 498-502. Prentice Hall, New Jersey.
McArthur, J. M., P. Ravenscroft, S. Safiullah, and M. F. Thirlwall. 2001. Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resources Research 37:109-117.
Newman, D. K., T. J. Beveridge, and F. M. M. Morel. 1997. Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl. Environ. Microbiol. 63:2022-2028.
Nickson, R., J. McArthur, W. Burgess, K. M. Ahmed, P. Ravenscroft, and M. Rahman. 1998. Arsenic poisoning in Bangladesh groundwater. Nature 395:338.
Santini, J. M., L. I. Sly, R. D. Schnagl, and J. M. Macy. 2000. A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological, and preliminary biochemical studies. Appl. Environ. Microbiol. 66:92-97.
Wilson R. 2000. Arsenic in specific countries. http://phys4.Harvard.edu/~wilson/arsenic_project_countries.html